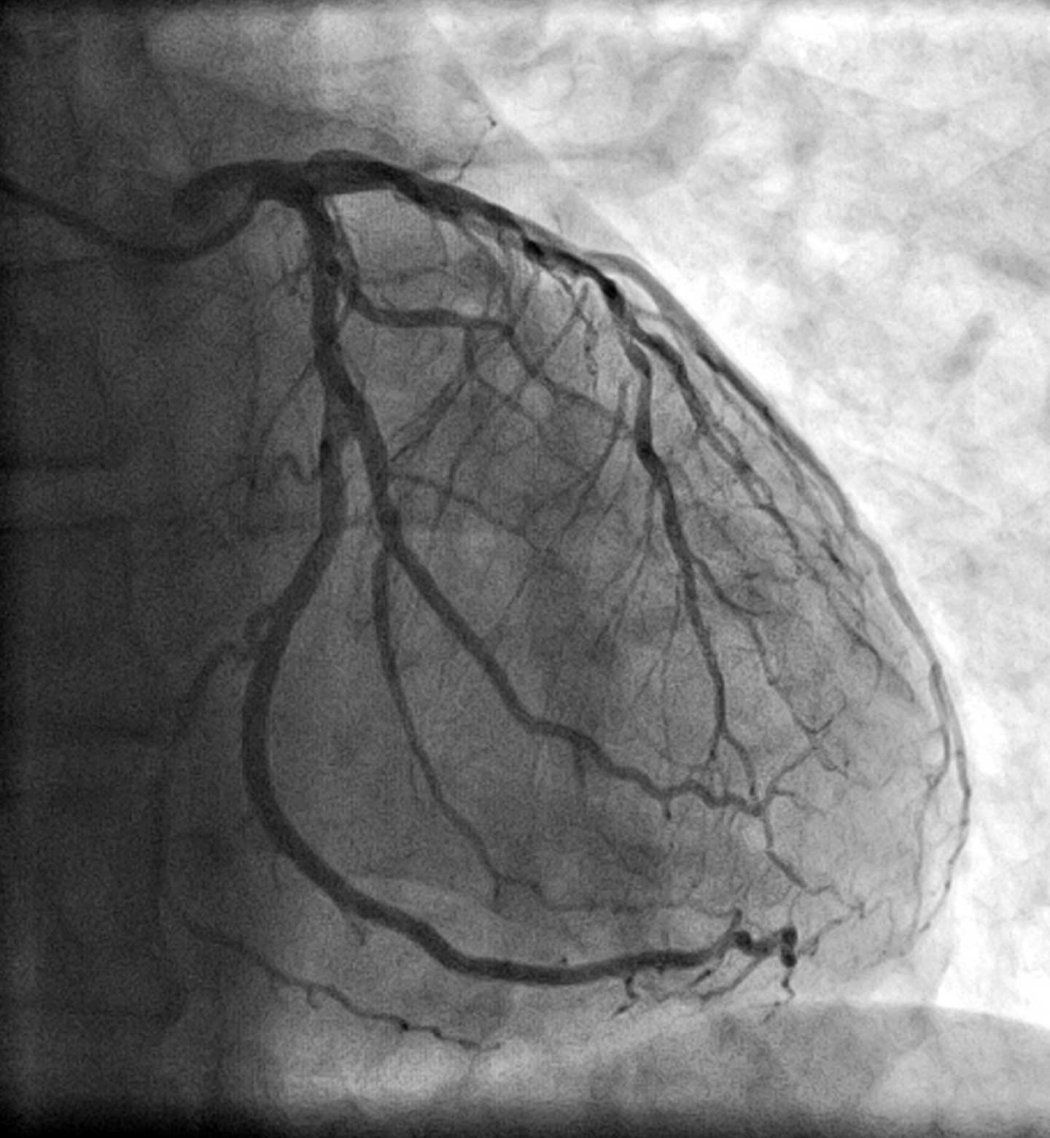
Coronary heart disease is the leading cause of adult death worldwide. In many cases, quantifying left ventricular ejection fraction (LVEF) – or how much blood the left ventricle pumps out with each contraction – is critical to optimize decision-making and treatment decisions, particularly for acute coronary syndromes like heart attacks or unstable angina.
Up until now, quantifying LVEF required invasive testing using a catheter that comes with its own risks – not ideal for patients already experiencing heart and stroke issues. But that might be about to change with the help of artificial intelligence and research by UCSF cardiologist Geoff Tison, MD, MPH, and Robert Avram, MD, a former UCSF research fellow now at the Montreal Heart Institute.
Do I understand correctly that you’re using artificial intelligence to analyze how much blood the heart is pumping in individual patients?
Well, Yes and No. Our recent research, published in JAMA Cardiology used deep neural networks, a type of AI algorithm called CathEF, to predict how much blood a heart was pumping using standard angiogram videos – x-ray images that visualize the inside of blood vessels, in particular arteries, veins and the heart chambers – with the goal of gaining new insights into possible patient treatment in critical situations.
Just to be clear, this was research to test the viability of the approach. We didn’t use it to influence the treatment of patients.
How can AI predict how much blood a heart is pumping?
We fed it coronary angiograms of more than 4,000 of patients along with corresponding transthoracic echocardiograms – or coronary ultrasound tests of 3,600 patients. Angiograms and echocardiograms are the standard diagnostic assessment for nearly all heart disease related decision-making, from medications to coronary bypass surgery, so just about everyone with heart and stoke issues has them done.
What was the CathEF AI looking for?
Using the angiograms and echocardiograms, we were able to establish a baseline. Then, we optimized CathEF to estimate reduced LVEF of less than or equal to 40%, indicating the need for additional clinical analysis and possible testing.
How did CathEF perform?
The results showed that CathEF accurately predicted LVEF with strong correlations to echocardiographic LVEF measurements, which is currently the standard noninvasive clinical approach to determine LVEF. The model was also externally validated in angiograms from the Ottawa Heart Institute.
CathEF performed well across different patient demographics and clinical conditions, including acute coronary syndromes and varying levels of renal function – patient populations that may be less well suited to receive the standard left ventriculogram procedure.
How does CathEF improve a cardiologists’ diagnostic arsenal?
Using AI leverages data that is already collected during every angiogram to provide critical information that is not currently available to clinicians. We believe this novel approach could be adapted to provide real-time LVEF information that informs clinical decision-making and may help to enable better outcomes for patients.
What could this mean for patients?
Presently, measuring LVEF during angiography requires an additional invasive procedure called ventriculography – where a catheter is inserted into the left ventricle and contrast dye is injected – which carries additional risks. It can increase the risk of arrhythmias or stroke to the patient, and so it’s less frequently performed. CathEF could help many patients avoid these risks while providing the information clinicians need to make treatment decisions and ultimately improving patient outcomes and quality of life.
What’s next for CathEF?
Although the algorithm was trained on a large dataset of angiograms and echocardiograms from UCSF and then separately validated in a dataset from the Ottawa Heart Institute, we are undertaking further research to test this algorithm at the point-of-care and determine its impact on the clinical workflow in patients suffering heart attacks.