A new variation of the CRISPR-Cas9 gene editing system makes it easier to re-engineer massive quantities of cells for therapeutic applications. The approach, developed at Gladstone Institutes and UC San Francisco (UCSF), lets scientists introduce especially long DNA sequences to precise locations in the genomes of cells at remarkably high efficiencies without the viral delivery systems that have traditionally been used to carry DNA into cells.
“One of our goals for many years has been to put lengthy DNA instructions into a targeted site in the genome in a way that doesn’t depend on viral vectors,” says Alex Marson, MD, PhD, director of the Gladstone-UCSF Institute of Genomic Immunology and senior author of the new study. “This is a huge step toward the next generation of safe and effective cell therapies.”
In the new paper published Aug. 25, 2022, in the journal Nature Biotechnology, Marson and his colleagues not only describe the technology but show how it can be used to generate CAR-T cells with the potential to fight multiple myeloma, a blood cancer, as well as to rewrite gene sequences where mutations can lead to rare inherited immune diseases.
“We showed that we can engineer more than one billion cells in a single run, which is well above the number of cells we need to treat an individual patient,” says first author Brian Shy, MD, PhD, a clinical fellow in Marson’s lab.
From Double- to Single-Stranded DNA
CRISPR-Cas9, a system that edits genes inside living cells, has been used as a basic research tool for the past decade. Increasingly, many clinician scientists are excited about the potential of CRISPR-Cas9 to generate living cell therapies.
With gene editing, one can turn off, delete, or replace a mutated, disease-causing gene, or boost the cancer-fighting activity of an immune cell, among other things. While the first therapeutic applications of CRISPR-Cas9 have recently entered clinical trials, the technology has still been limited by the challenge of safely making large quantities of correctly edited cells.
Traditionally, researchers have relied on viral vectors – the shells of viruses without their disease-causing components – to carry the DNA (called the DNA template) used for gene therapy into cells. However, manufacturing bulk amounts of clinical-grade viral vectors has been a major bottleneck in getting cell therapies to patients. In addition, researchers can’t easily control where traditional viral vectors insert genes within the genome.
“Using viral vectors is expensive and resource intensive,” says Shy. “A major benefit of a non-viral approach to gene engineering is that we’re not as limited by cost, manufacturing complexity, and supply chain challenges.”
In 2015, Marson’s group – in collaboration with the lab of CRISPR pioneer Jennifer Doudna, PhD – first showed that they could insert short DNA templates into immune cells without viral vectors, using an electrical field that makes cells’ outer membranes more permeable. By 2018, they developed a method to cut and paste longer DNA sequences into immune cells with CRISPR.
Then, in 2019, the researchers discovered that by also using a modified version of the DNA templates that can bind to the Cas9 enzyme – the same protein that acts as molecular scissors during CRISPR gene editing – they could deliver the new sequences to the targeted genome site more efficiently.
However, more work was required to improve the yield of successfully engineered immune cells and to make the process compatible with the manufacturing of future cell therapies. Those goals motivated the team’s current study.
DNA can exist in single or double strands (like opposing pieces of Velcro), and Cas9 attaches to double-stranded DNA. The researchers quickly discovered that high levels of double-stranded DNA template can be toxic to cells, so the method could only be used with low quantities of template DNA, leading to a low efficiency.
The team knew that single-stranded DNA was less toxic to cells, even at relatively high concentrations. So, in the new paper, they describe a method to attach the modified Cas9 enzyme to a single-stranded template DNA, by adding just a small overhang of double-stranded DNA at the ends.
“This gives us a balanced, best-of-both-worlds approach,” says Marson.
Single-stranded template DNA could more than double the efficiency of gene editing compared to the older, double-stranded approach. And the double-stranded ends of the molecules let researchers use Cas9 to enhance the delivery of non-viral vectors into cells.
“This technology has the potential to make new cell and gene therapies faster, better, and less expensive,” says Jonathan Esensten, MD, PhD, an author of the new work who is an assistant professor of laboratory medicine at UCSF and an affiliate investigator at Gladstone.
A Path to the Clinic
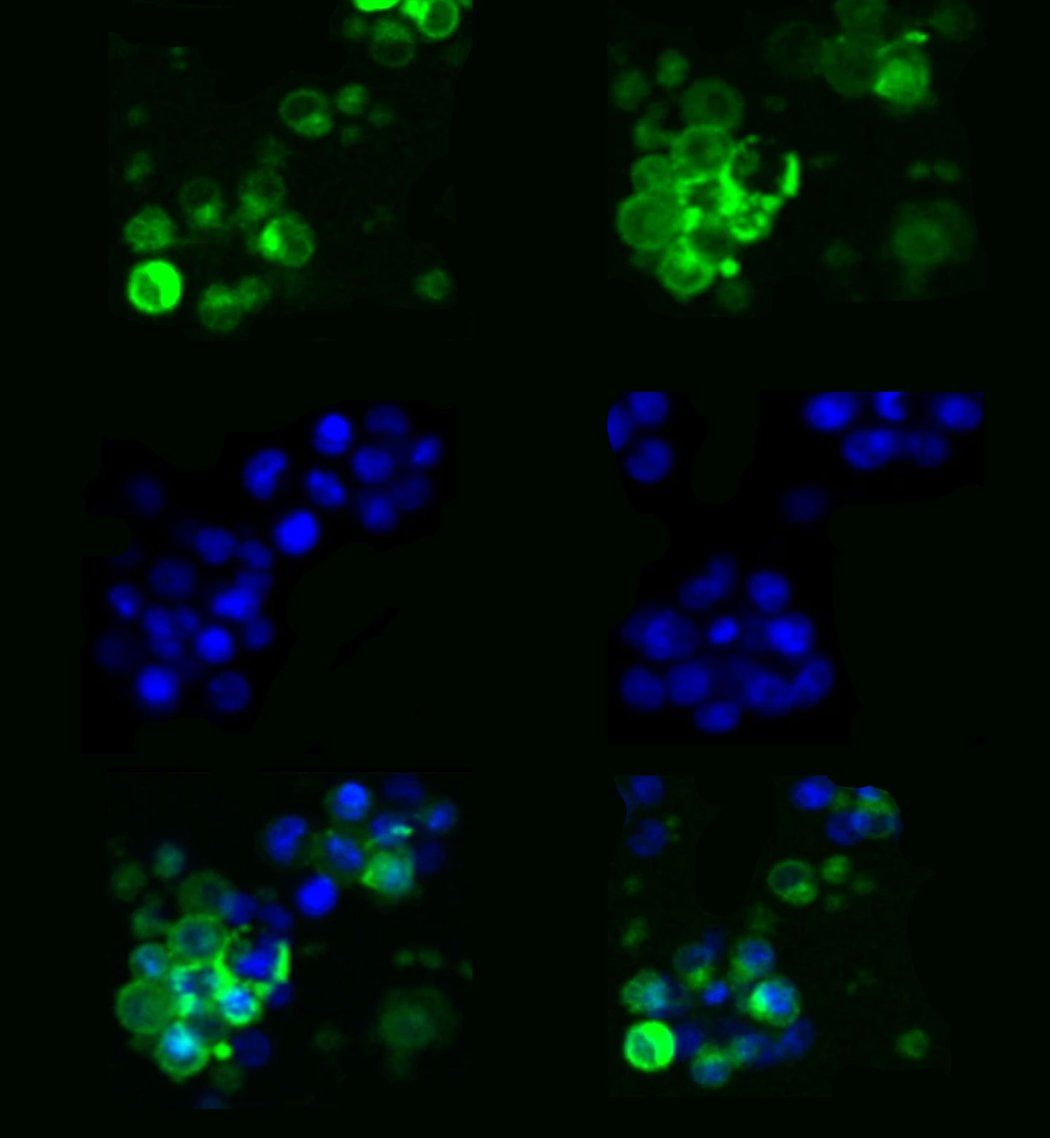
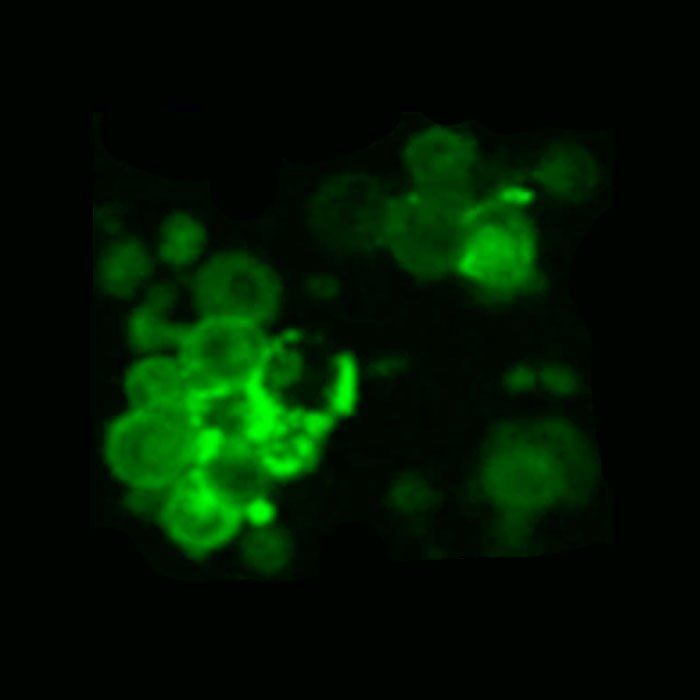
In the study, researchers used the new DNA template to generate more than a billion CAR-T cells that target multiple myeloma. CAR-T cells are immune T cells genetically modified to effectively fight specific cells or cancers. With the new single-stranded, Cas9 directed templates, approximately half of all T cells gained the new gene and, as a result, were converted to CAR-T cells.
We showed that we can engineer more than one billion cells in a single run, which is well above the number of cells we need to treat an individual patient.
“We knew that targeting the DNA templates to a specific location in the genome, called the TRAC site, would improve the anti-tumor potency of CAR-T cells,” says Justin Eyquem, PhD, a co-author of the new paper, assistant professor of medicine in the Division of Hematology and Oncology at UCSF, and affiliate investigator at Gladstone. “This new non-viral approach enables us to achieve that targeting much more efficiently, which will accelerate the development of the next generation of CAR-T cell therapies.”
In addition, the researchers showed that their approach could, for the first time, replace in their entirety two genes associated with rare genetic immune diseases, the IL2RA and the CTLA4 genes.
In the past, scientists had shown they could replace small sections of the IL2RA gene where particular patients have mutations. Now, Marson’s team proved that they can replace the whole IL2RA and CTLA4 genes at once – a “one size fits all” approach that could treat many patients with different mutations in these genes, rather than having to generate personalized templates for each patient’s mutation. Nearly 90 percent of the cells treated with this gene engineering approach gained the healthy versions of the genes.
The researchers are now seeking approval to advance clinical trials using non-viral CRISPR technology in both CAR-T cell therapy and the treatment of IL2RA deficiency.
Other authors are Vivasvan S. Vykunta, Alvin Ha, Alexis Talbot, Theodore L. Roth, David N. Nguyen, Yan Yi Chien, Franziska Blaeschke, Eric Shifrut, Shane Vedova, Murad R. Mamedov, Jing-Yi Chung, and Ruby Yu of the Gladstone-UCSF Institute of Genomic Immunology; Wolfgang G. Pfeifer and Carlos E. Castro of The Ohio State University; Hong Li and Lumeng Ye of GenScript Biotech; and David Wu, Jeffrey Wolf, and Thomas G. Martin of UCSF.
The work was supported by the NIH grants P01AI138962, P01AI155393, L40AI140341, K08AI153767, L30TR002983, F30DK120213, and other sources.
About UCSF: The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area. UCSF School of Medicine also has a regional campus in Fresno. Learn more at ucsf.edu or see our Fact Sheet.
About Gladstone Institutes: To ensure our work does the greatest good, Gladstone Institutes focuses on conditions with profound medical, economic, and social impact – unsolved diseases. Gladstone is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. It has an academic affiliation with the University of California, San Francisco.