Resilience to ALS Due to Synaptic Safety Mechanism
Self-Corrective Mechanism at Synapse Doubles Lifespan for Mice with ALS, Suggests New Approach to Therapy for Human Brain Diseases
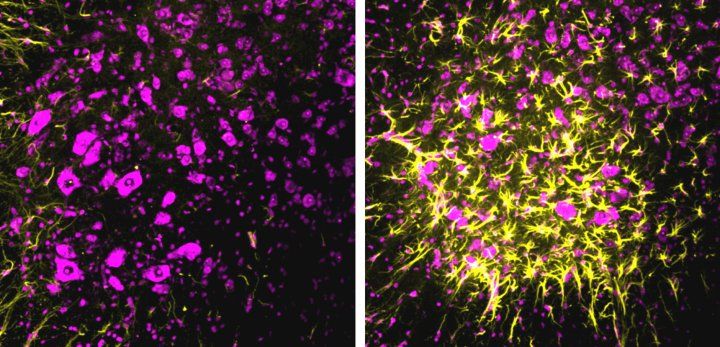
Spinal cord neurons (purple) in mice suffer degeneration (yellow) when a synaptic repair mechanism is removed. Image by Graeme Davis laboratory
A common feature of neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease) is the progressive loss of synapses – the anatomical sites of communication between brain cells – throughout the brain and spinal cord. Typically, synapse loss becomes pervasive before the outward appearance of symptoms of disease, such as memory loss or paralysis. The fact that there must be extensive synapse loss before brain function begins to seriously decline suggests that the nervous system maintains a deep functional reserve that keeps everything working normally until the damage passes a tipping point and the brain’s resilience begins to break down.
But how exactly does this functional reserve confer resilience in the face of ongoing brain degeneration? Could differences in this reserve explain why some individuals with ALS decline and die within months, while others – like astrophysicist Steven Hawking – live for decades? And could a treatment that boosts this functional reserve help more patients survive and prosper as long as Hawking?
In a new study published May 6, 2020, in Neuron, UC San Francisco neuroscientist Graeme Davis, PhD, and his team have identified a powerful self-corrective mechanism within synapses that is activated by neurodegeneration and acts to slow down disease progression in animal models of ALS. Selectively eliminating this self-corrective mechanism dramatically accelerated progression of ALS in mice, shortening their lifespan by 50 percent.
“Our data provide the first evidence that neurodegeneration kick-starts a self-corrective response that can keep the synapse between nerve and muscle working correctly, even though the disease process has already begun to nibble away at the synapse bit by bit, day by day. We also show for the first time that this self-corrective mechanism is amazingly potent – keeping mice with ALS alive twice as long as they would otherwise survive,” said Davis, the Morris Hertzstein Distinguished Professor of Medicine in the UCSF Department of Biochemistry and Biophysics and member of the UCSF Weill Institute for Neurosciences, the UCSF Kavli Institute for Fundamental Neuroscience, and the Eli and Edythe Broad Institute for Regenerative Medicine and Stem Cell Research at UCSF.
Davis has doggedly pursued the molecular underpinnings of self-corrective brain plasticity for decades, and his group is now translating their initial gene discovery efforts in mouse models of human diseases that include ALS, Alzheimer’s and epilepsy. Their goal is to leverage their most recent discoveries into a drug that boosts the brain’s self-corrective capacity, creating a greater functional reserve for people with ALS and other neurodegenerative diseases.
“Self-corrective plasticity could confer resilience to neurodegeneration of any kind and any cause, rather than being specific a single disease such as ALS,” Davis said. “I believe that we can improve the brain’s functional reserve, bringing everyone up to peak capacity and potentially extending lifespan across neurodegenerative diseases."
Authors: Joining Davis, senior and corresponding author, were Brian Orr and Anna Hauswirth of UCSF, co-lead authors of the study. Other authors were Barbara Celona, Richard Fetter, Giulia Zunino, and Brian Black of UCSF; Evgeny Kvon and Yiwen Zhu of Lawrence Berkeley National Laboratory (LBNL); and Len Pennacchio of LBNL and the U.S. Department of Energy Joint Genome Institute.
Funding: The research was supported by the US National Institutes of Health (NIH) (NINDS R35NS097212, NIH HL146366, NHGRI R01HG003988, NHGRI 5K99HG009682) and the ALS Association (17-IIP-358), with research conducted at the E.O. Lawrence Berkeley National Laboratory and performed under Department of Energy Contract DE-AC02-05CH11231.
Disclosures: The authors declare no competing financial interests.
The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. It includes UCSF Health, which comprises three top-ranked hospitals, as well as affiliations throughout the Bay Area.