Kaveh Ashrafi, PhD, raves rhapsodically about a dirt-digging, bacteria-eating roundworm. The worm is just one millimeter long. To the uninitiated, Ashrafi's enthusiasm might seem outsized.
But young postdoctoral scientists of all stripes - neuroscientists, geneticists, endocrinologists - come to his lab to discover what the worm can show us about why most of us are getting fat. The worm,
C. elegans, is an apt subject for studies of fat storage, metabolism, feeding and foraging, Ashrafi says.
Fat is favored for long-term energy storage. Fat is more energy-dense than the other major nutrient sources, proteins and carbohydrates. Some fat cravers might also suspect that fat is a mind-altering drug.
It would be a stretch to say that a worm has a mind. But Ashrafi has confirmed that the nervous system of
C. elegans, similar to the nervous system of a human, responds to internal energy reservoirs as well as to external signals about food availability.
Step by step, Ashrafi is tracking down the specific cells and molecules involved in evoking sophisticated signaling and orchestrated responses by the worm brain in response to these stimuli. His work is shedding a clear light on how sensory cues are translated into changes in feeding behavior and fat metabolism.
New Drug Strategies for Weight Loss
To much fanfare and pharmaceutical company interest, researchers identified the hormone leptin in mice more than a decade ago. Studies showed that an increase in leptin secretion from fat cells when energy stores are plentiful can lead to less eating and to an increase in energy expenditures.
But leptin failed as a long-term drug in both mice and men. Not enough was known about the body's feedback mechanisms and the effects of perturbing the system.
Despite the failure, the leptin discovery spurred new research that led to new drug development and clinical trials. However, the success of weight loss drugs remains elusive. For instance, the drug Acomplia, sold in Europe, was not approved for US sale after it recently was linked to increased suicide risk among depressed users.
Worms don't have leptin. Still,
C. elegans may be the key to getting a better handle on biological mechanisms and feedback that affect how fat we get, Ashrafi says. The research could lead to new ideas for weight loss drugs that may be effective with fewer side effects.
"To really understand the problem, you have to be able to dissect it all the way from metabolism to behavior," Ashrafi says. "The worm is pretty much perfect for this. It has the right balance of simplicity and complexity."
959 Worm Cells Mimic Trillions
Cells in a human number many trillions. By contrast,
C. elegans has 959. But Ashrafi and others have found that although
C. elegans has few cells, these cells are sufficient to endow the worm with a suite of important behaviors and biological feedback responses that it shares with humans.
In part, that's because - despite its few cells -
C. elegans possesses many cell types. For instance, its "brain" consists of just 302 nerve cells, but among these are 118 different types of nerve cells, Ashrafi says.
In addition, roundworms, like humans, have many genes directing the production of many proteins. These proteins drive myriad events, including metabolism, signaling and the production of additional molecules that the worm uses. Each
C. elegans cell contains roughly 20,000 protein-encoding DNA sequences. The number of these DNA sequences in humans is roughly 30,000 - the same order of magnitude.
So, while the worm brain pales in a side-by-side comparison with the human brain, the range of neurotransmitters and other nerve-signaling molecules coursing through the worm nonetheless is comparable to what is found in humans, Ashrafi says.
For worms, using nerves, hormones and whatever feedback mechanisms they can muster to control energy stores makes sense, just as it does for more highly evolved creatures like ourselves.
"Most organisms cycle between feast and famine," Ashrafi says. "In response, organisms have evolved sophisticated ways of storing energy. There is a tremendous amount of conservation through evolution in terms of how these systems are organized."
Indeed, Ashrafi's comparison of human and worm genes suggests that the worm's ways of controlling energy storage are likely to operate in humans too.
Worming a Way Toward Understanding
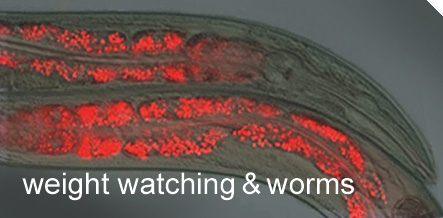
With the right tools, dissecting and otherwise manipulating worm parts are fairly easy. Researchers also can see right through the worm. Even without dissection, by using fluorescently tagged molecules, Ashrafi can trace fat storage and determine whether specific genes are turned on or off -
C. elegans becomes a glowworm for a new era of biotech.
In the worm, Ashrafi can quickly and precisely switch off individual genes. He does this to see which genes affect behaviors and biochemistry related to feeding, fat storage and energy metabolism. The researchers just feed the worms their staple food,
E. coli bacteria, dosed with interference RNA. The RNA is coded to switch off the worm gene of interest once it gets inside.
Ashrafi's studies of worm genetics, neuroscience and behavior support the idea that there may be more than one way to trim the fat. Studies of the nervous system alone suggest more than one approach.
"We have discovered that the nervous system of the worm can regulate fat metabolism independently of feeding behavior. So, fat content is not simply a by-product of feeding behavior," Ashrafi says. "I wouldn't be surprised if similar regulatory mechanisms operate within us, as well." Ashrafi has published some of his most striking recent findings in the June 4, 2008, edition of the scientific journal
Cell Metabolism.
From Worm to Human and Back Again
Using RNA interference, Ashrafi already has identified about 400 genes that affect energy intake, expenditure and storage in
C. elegans. "The majority of genes that we found in worms have obvious human counterparts," he says. "Most of them have not previously been shown to have similar roles in humans, but I'm comfortable betting that a number of them will prove to be important."
Ashrafi is collaborating with UCSF physician Christian Vaisse, MD, PhD. Vaisse's research focuses on human genes that may be involved in obesity and diabetes. Ashrafi already has been seeing what more can be learned about the operations of some of these important human genes, including rare mutations, by studying them in
C. elegans. Now, the researchers also can begin to investigate how previously unsuspected genes that affect energy balance in
C. elegans might affect humans too. Common variations in some of these genes may influence who gets fat.
"Our baseline, resting metabolism accounts for roughly 70 percent of the energy we expend, so the difference between you and me has largely to do with proteins that make cells run," Ashrafi says. "It's not too hard to imagine that individual genetic differences that affect how efficiently cells run could be important in determining susceptibility to weight gain.
"But at the end of the day, we are experimental scientists. We don't know until we do the experiments. The nice thing about the worm is that you can do lots of experiments relatively quickly."
Serotonin Regulates C. elegans Fat and Feeding
Through Independent Molecular Mechanisms
Supriya Srinivasan, Leila Sadegh, Ida C. Elle, Anne G.L. Christensen,
Nils J. Faergeman and Kaveh Ahrafi Cell Metabolism , vol. 7, issue 6, June 4, 2008
Abstract | Full Text |
Related Links:
Are We Destined to Gain Weight?
UCSF Today, April 17, 2006
Obesity and the Regulation of Fat Metabolism
Kaveh Ashrafi
WormBook, the Online Review of
C. elegans Biology
Kaveh Ashrafi Lab at UCSF
Eating and Weight Gain Not Necessarily Linked, Study Shows
UCSF News Release, June 3, 2008
Program in Metabolism, Obesity and Type 2 Diabetes
UCSF Diabetes and Endocrinology Research Center