By Jeffrey Norris
Thanks to rarely talked-about recent advances in radiation treatments, young children are surviving cancers that would have been incurable a decade ago. One strategy that is helping to boost survival is radiation treatment provided at the time of surgery. UCSF and fewer than a dozen other medical centers nationwide are at the forefront in advancing this mode of treatment, called intraoperative radiation therapy (IORT).
With IORT, patients do not have to return for successive rounds of therapy after surgery. Those who receive the most common standard alternative, external beam radiation therapy (EBRT), typically receive treatment five days a week for several weeks. Another advantage of IORT over EBRT is that the surgical team is better able to position normal tissue out of the path of beamed radiation. This lessens the risk that healthy tissue will be damaged, and allows oncologists to deliver higher doses of radiation to tumors.
Survival and Side Effects
Like chemotherapy, radiation treatments can have side effects. IORT involves giving a high radiation dose over a short period of time - typically 10 to 15 units, called grays, compared with an average cumulative dose of 20 to 60 grays for EBRT. IORT has very limited short-term side effects.
 |
Katherine Matthay |
But aggressive treatments also have long-term side effects. With growing numbers of patients outliving cancer, physicians are learning more about long-term risks, such as scarring and fibrosis. Clinical researchers now are on the lookout for harbingers of future complications of treatment even as they aim to deliver more powerful, better targeted blows to tumors.
IORT May Be Lifesaving in Neuroblastoma
UCSF is a leading referral center for the treatment of pediatric cancers. UCSF radiation oncologists are pioneering the use of IORT in the treatment of the nation's most difficult cases of neuroblastoma, a leading childhood cancer. Neuroblastoma is a cancer that arises in nerve tissue that resembles residual embryonic tissue. It is the most common solid tumor that develops outside the brain in children. Neuroblastoma rarely arises in children past age 10. It most often occurs in the adrenal gland atop the kidneys, or in nerve fibers of the chest or abdomen.
For two decades, oncologists have treated the most aggressive cases of neuroblastoma with surgery, high doses of chemotherapy and some form of radiation. These high-dose treatments kill tumor cells more effectively. But the treatments also result in toxicities in many normal tissues, such as the death of bone marrow cells that make blood and immune cells. Thanks to these high-dose combinations of radiotherapy and chemotherapy, survival among neuroblastoma patients has improved greatly over the past two decades. Even so, among the 45 percent with high-risk tumors, the worst prognosis, only about one in three lives free of cancer regrowth for three years or more.
Since the mid-1980s, oncologists at UCSF have been pioneering the use of IORT, with or without EBRT, as the radiation component of therapy. To generate high-energy radiation for treatment, UCSF surgeons use a mobile linear accelerator called a Mobetron right in the operating room. Patients don't have to be moved during surgery to receive treatment.
Amy Gillis, a fourth-year UCSF resident in radiation oncology, recently worked with UCSF radiation oncologist Daphne Haas-Kogan and pediatric oncologist Katherine Matthay to review 31 consecutive cases of high-risk abdominal neuroblastoma treated with IORT at UCSF. Gillis, who presented findings at the annual meeting of the American Society for Therapeutic Radiation and Oncology, estimated that nearly one-half of patients survived, or were on track to survive, three years or more without cancer regrowth. The best results occurred when tumor location permitted surgeons to remove all visible cancer.
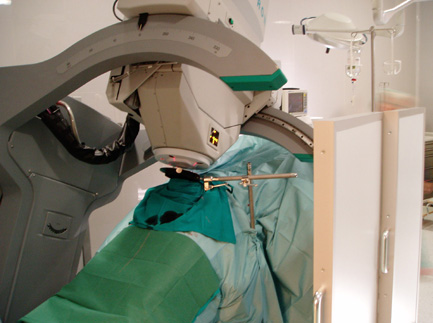 |
UCSF oncologists have pioneered the use of radiation treatment at the time of cancer surgery. In the operation room, a movable linear accelerator - called a Mobetron - makes it possible to treat patients without moving them. |
Neuroblastoma often encircles major blood vessels. Some patients in the study developed major blood vessel constrictions, and two of them died as a result. Gillis notes that it was not clear to her and her colleagues whether these effects were due to tissue damage from treatment or were caused by the cancer itself. Nonetheless, Gillis says, despite the evidence for improved neuroblastoma treatment outcomes with IORT, she and her colleagues recommend that "extreme caution be used in delivering IORT to large blood vessels in younger children."
Novel Radiotherapy in Drug Form
IORT is not the only newer mode of radiation therapy being studied in neuroblastoma. Matthay leads clinical trials through the nationwide consortium New Approaches to Neuroblastoma Therapy.
Matthay has pioneered studies of a treatment called
131I-MIBG in high-risk cases of drug-resistant, metastatic or recurrent neuroblastoma. A component of
131I-MIBG is a gradually decaying radioisotope of iodine, which acts as a radiotherapy. The injected drug specifically targets neuroblastoma and related cells, and also can be used to visualize tumor tissue with scans.
Matthay and her consortium collaborators have shown that
131I-MIBG can effectively be combined with standard chemotherapy to improve the duration of survival without neuroblastoma regrowth, even in patients whose cancers have grown and spread despite prior treatments.
(This story first appeared in the Summer 2007 issue of the
UCSF Comprehensive Cancer Center Report.)
Image/IntraOp Medical Corp.
Related Links:
Matthay Named to New Endowed Chair in Pediatric Translational Research
UCSF Today, April 30, 2007
For Heroic Cancer Kids, Life Is a Trial
UCSF Magazine, August 2004
New Mobile Electron System in Use at UCSF
UCSF Daybreak, March 10, 1998
New Approaches to Neuroblastoma Therapy
UCSF Comprehensive Cancer Center
