Looking Beyond DNA to See Cancer with New Clarity
Mapping How Mutated Proteins Interact Reveals Previously Unseen Drug Targets
Researchers at UC San Francisco and UC San Diego have mapped out how hundreds of mutations involved in two types of cancer affect the activity of proteins that are the ultimate actors behind the disease. The work points the way to identifying new precision treatments that may avoid the side effects common with much current chemotherapy.
“This is an entirely new way to do cancer research,” said Nevan Krogan, PhD, director of the UCSF Quantitative Biosciences Institute who is co-senior author, along with Trey Ideker, PhD, professor at UC San Diego School of Medicine, on a set of three related papers that appear Sept. 30, 2021, in Science. “We realized we need another way to look at cancer that takes it a step beyond DNA.”

Nevan Krogan, PhD, director of the UCSF Quantitative Biosciences Institute who is co-senior author, along with Trey Ideker, PhD, professor at UC San Diego School of Medicine, on a set of three related papers that appear Sept. 30, 2021, in Science. Photo by Susan Merrell
Krogan and Ideker look to proteins, which carry out the vast majority of functions in the body – and which take on a collection of forms that far outnumber our genes, providing a much more expansive view of the activity underlying cancer.
“We’re elevating the conversation about cancer from individual genes to proteins, allowing us to look at how the varying mutations we see in patients can have the same effects on protein function,” said Ideker, noting that this work represents new technological capacity to explain the effects mutations in a more precise way. “We’ve produced the first map looking at cancer through the lens of interactions between proteins.”
The effort to map these effects, dubbed Cancer Cell Map Initiative (CCMI), is revealing, on a monumental scale, genetic patterns and organizing principles that underlie the disease and potential new ways to tackle it.
The team’s new studies describe the approach in detail, and highlight findings when it was applied to breast cancer and cancers of the head and neck.
Looking Beyond Gene Mutations to Protein Disruptions
Our genes contain instructions for building proteins, which then interact with other proteins, almost always in large groups called complexes. These protein complexes often regulate an activity or turn a function on or off. If the underlying gene has a mutation, the resulting protein complexes will as well.
These gene mutations can affect how well the resulting protein complexes do their job. For example, a particular interaction between two proteins might be crucial to repairing damaged DNA. If the mutated version of one of those proteins is shaped differently than normal, it may not interact correctly with the other protein, and the DNA might not get repaired, leading to cancer.
Currently, there are a small number of mutated genes that physicians look at as biomarkers – quantifiable indicators, such as the presence of a particular molecule, that denote a condition in the body – to help them determine whether a particular cancer drug might benefit a patient, said Ideker.
“The problem is that we’ve only found a few genes that we can work with in this way to help guide prescription of an FDA-approved drug,” he said. “Our studies provide a new definition of biomarkers based not on single genes or proteins but on the large, multi-protein complexes.”
Mapping Protein Mutations
There is a subset of genes that are commonly mutated in cancer, and each of these genes can be mutated in hundreds of different ways, said Krogan, who is also a senior investigator at Gladstone Institutes. In addition, the function of a particular protein may be different in different types of cells, so a mutation in a breast cancer cell might have different effects on protein complexes than that same mutation’s effect in a cell of the throat.
CCMI’s goal was to map the constellation of protein complexes formed by about 60 genes commonly involved in either breast cancer or cancers of the head and neck, and to see what each looked like in healthy cells. Alongside that effort, he created maps of how protein complexes are affected by hundreds of gene mutations in two cancerous cell lines.
Doing so presented a formidable computational challenge. The CCMI collaboration allowed the team to use advanced and novel data analysis to reveal not only whether the mutation affected interactions between proteins, but to what extent.
“That kind of detail shows us how well an existing drug might work, or explain why it doesn’t,” Ideker said.
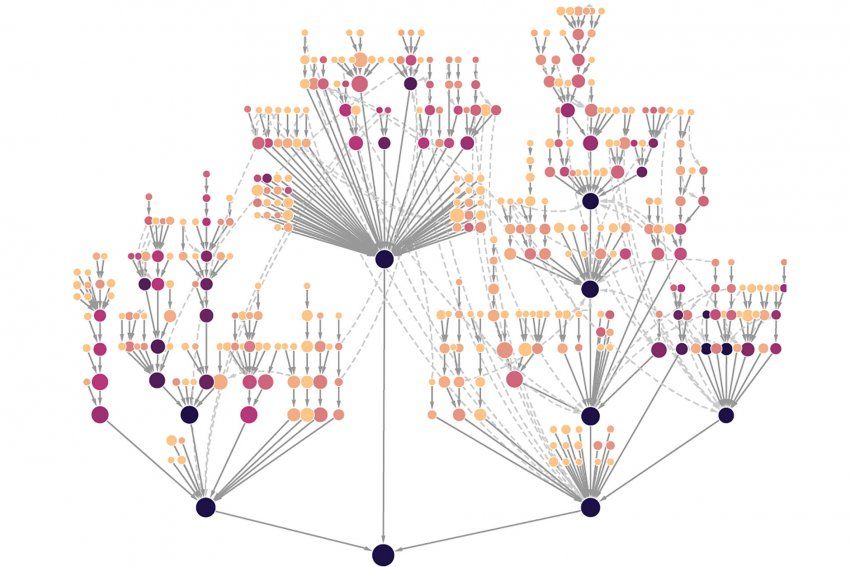
Each node represents a protein system carrying out cellular functions such as mobility or immune signaling. Nodes farther out on the branches represent systems with few proteins and highly specialized processes, while those closer to the root have many proteins and correspond to generalized processes. Darker colored systems, and their subsystems, are under selection in more tumor types. Image by Krogan lab
The most powerful aspect of these extensive protein interaction maps is that they can shed the same light on many other conditions, the authors said. For example, they are also working on similar studies of protein interactions in psychiatric and neurodegenerative disorders, as well as infectious disease.
Ideker and Krogan see the CCMI collaboration as the real source of strength behind this approach.
“We’re not only making connections between different genes and proteins but between different people and different disciplines,” Krogan said. Those collaborations have built up an infrastructure that allows the team to integrate an array of information and push the boundaries of what’s possible in applying data science to complex diseases.
“We’re in the perfect position to take advantage of this revolution on every level.” said Krogan. “I couldn’t be more excited than I am right now. We can do such damage to cancer.”
Krogan and Ideker are co-senior authors on all three papers. First authors are Danielle Swaney, PhD, and Minkyu Kim, PhD, MS, of UCSF and Fan Zheng, PhD, and Marcus Kelly, PhD, of UCSD For further author information, please see the studies.
The research was supported by grants from the National Cancer Institute (U54 CA209891, U54CA209988, 5F30CA236404-02) and the National institutes of Health (F32 CA239336, R50 CA243885, S10 OD026929) as well as other public and philanthropic sources.
About UCSF: The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area.
About QBI: The Quantitative Biosciences Institute (QBI) fosters collaborations across the biomedical and the physical sciences, seeking quantitative methods to address pressing problems in biology and biomedicine. Motivated by problems of human disease, QBI is committed to investigating fundamental biological mechanisms, because ultimately solutions to many diseases have been revealed by unexpected discoveries in the basic sciences.