Researchers Identify Lynchpin to Activating Brown Fat Cells
UCSF Study Sheds Light on Fat-Burning Process That Could Help in Fighting Obesity
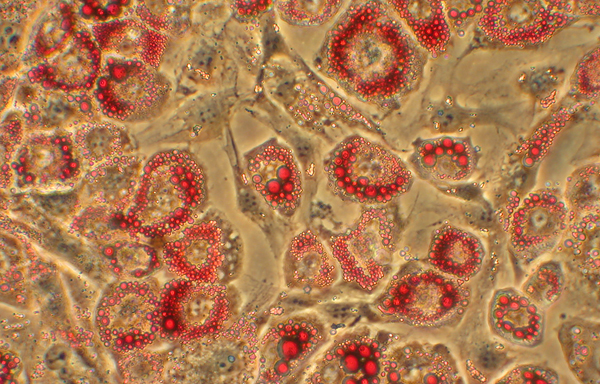
Fat cells are shown under a microscope.
Researchers at the University of California, San Francisco (UCSF) have identified the lynchpin that activates brown fat cells, which burn fat molecules instead of storing them, making them the focus of pharmaceutical research aimed at fighting obesity.
The importance of brown fat in burning calories to generate heat in humans — as distinct from other mammals such as mice and bears — has only been appreciated in recent years. In humans, brown fat was until recently thought to be present only in infants. Scientists now know that adult humans possess small but significant deposits of brown fat — to go along with the all-too-obvious white fat that especially plagues the growing ranks of the obese.

Yuriy Kirichok, PhD
Brown fat can be activated by cold temperatures, and other research suggests that other stimuli, such as overeating, might also activate brown fat. Once activated, brown fat cells essentially causes the energy contained within fat molecules to be converted to heat — a process understood as a way for hibernating bears to maintain body temperature and for small mammals with lots of exposed body surfaces to keep warm.
As described in the October 12 issue of the scientific journal Cell, Yuriy Kirichok, PhD, associate professor of physiology at UCSF — working with postdoctoral fellow Andriy Federenko and research specialist Polina Lishko, PhD, now an assistant professor at UC Berkeley — discovered how a protein triggers the biochemical mechanism responsible for fat burning in the brown fat cell.
The new discovery suggests it may be possible to come up with a molecule that could keep the mechanism in the “on” position, to increase fat burning in the body, said Kirichok, the Jack D. and DeLoris Lange Endowed Chair in Systems Physiology at UCSF.
Whether such a strategy would be practical for weight control, however, is unclear.
Uncoupling protein 1 (UCP1), the focus of the current study and a protein long known to be required for brown fat’s heat-generating capability, is only activated in brown fat, Kirichok said. This specificity may make the protein appear to be a good target, but the feedback generated within the fat cell in response to a drug might prove to be complicated, he said.
Regardless, the techniques Kirichok developed and used in this study should prove useful in investigating other key proteins that affect energy metabolism within cells, he said.
“Low levels of brown fat correlate with obesity,” Kirichok said. “We have shown how fatty acids attach directly to UCP1 and help it to break down an electrical potential across the mitochondrial membranes, causing the cell to burn more fat and to generate heat in order to regenerate this potential.”
Understanding the Fat-Burning Process
Within every cell there are organelles called mitochondria that serve as the cell’s power plants, converting food energy into ATP — the cell’s preferred fuel — through the metabolic process known as oxidative phosphorylation.
In oxidative phosphorylation, the chemical energy from fatty acids is used to create the voltage gradient across the mitochondrial membrane, and the potential energy of the voltage gradient then is used to make ATP.
Similar to the way a nine-volt battery left in your pocket might be bridged by coins and the battery wastefully run down while producing heat, UCP1 acts something like a short circuit that runs down the electrical potential normally generated across mitochondrial membranes by oxidative phosphorylation.
To a greater degree than other kinds of cells, including white fat, brown fat is chock-full of mitochondria. The abundance of mitochondria contributes to the cells’ brown hue. However, in the mitochondria of brown fat, ATP production takes a back seat. Instead, the UCP1 molecules embedded within the mitochondrial membranes of these cells dissipate the mitochondrial electrical potential and drive the consumption of fat and its conversion to heat, not ATP.
To probe the long-baffling link between fatty acids and UCP1 within the mitochondria of brown fat, Kirichok’s team used a highly refined version of a technique called “patch clamping” to track electrical currents. This technique allows researchers to record electrical activity and currents from a single mitochondrion. Kirichok and Lishko previously used the technique to identify a key event in the fertilization of egg by sperm in humans.
The role of UCP1 in dissipation of the mitochondrial electrical potential has been known for two decades, Kirichok said. In addition, long-chain fatty acids derived from food have long been known to be required for activation of UCP1 and thermogenesis. But it has remained unclear how the presence of fatty acids is linked to the functioning of UCP1.
With the patch clamp technique as refined by Kirichok, the UCSF researchers were able to directly track changes in electrical currents moving across mitochondrial membranes containing UCP1 under different experimental conditions, and to make inferences about what was happening biochemically. They concluded that UCP1 does not function as a simple channel to pass electrically charged ions. Instead the protein captures fatty acids and employs them to transport positively charged hydrogen ions (protons) into the mitochondria.
As more protons enter the mitochondria via UCP1, the electrical potential across the inner mitochondrial membrane dissipates. Additional cycles of oxidative phosphorylation and fat burning then are required to restore the electrical potential, Kirichok explained. During this process heat is generated.
Thermogenesis by brown fat in response to cold also involves the nervous system. The neurotransmitter molecule norepinephrine attaches to a receptor on the brown fat cell surface, which sets off a chain of events inside the cell that includes the breaking down of triglycerides from foodstuffs into the fatty acids needed by UCP1.
Given their key role in maintaining the vitality of a cell, mitochondria increasingly have become the focus of researchers who are curious about how they might malfunction in various diseases. Kirichok said he intends to continue using the patch clamp to investigate how energy and metabolism are controlled within mitochondria.
Top image by Shingo Kajimura
 Read
Read